RJPS Vol No: 15 Issue No: 4 eISSN: pISSN:2249-2208
Dear Authors,
We invite you to watch this comprehensive video guide on the process of submitting your article online. This video will provide you with step-by-step instructions to ensure a smooth and successful submission.
Thank you for your attention and cooperation.
Chetan Savant*1, Venkatrao Kulkarni1 , Prasanna Habbu1 , Preeti Kulkarni1 , Muhammed Majeed2 , Mahadeva Nayak2
1SET’s College of Pharmacy, Dharwad - 580002, Karnataka, India.
2R&D Sami Labs Limited, Peenya Industrial Area, Bangalore - 560058, Karnataka, India.
*Corresponding author:
Mr. Chetan Savant, Research Scholar, PG Department of Pharmacology, SET’s College of Pharmacy, Dharwad, Karnataka-580008. Email: chetan.savant@yahoo.com Affiliated to Rajiv Gandhi University of Health Sciences, Bengaluru, Karnataka.
Received date: September 18, 2020; Accepted date: December 14, 2020; Ahead of Print

Abstract
Generation of reactive oxygen species (ROS) occurs in the normal cell during metabolism in animals. Over production of ROS in the animals causes oxidative stress that leads to oxidative DNA damage which is implicated in the pathogenesis of numerous diseases. Since antioxidant mechanism plays vital role in the pharmacotherapy of different disorders, the present investigation aims to check the synergistic/antagonistic antioxidant effects of standardised extracts of Tinospora cordifolia (Willd) (TCE), Terminalia arjuna (Roxb) (TAE), Ocimum sanctum (Linn) (OSE) in combinations by DPPH Scavenging Activity, hydroxyl radical scavenging activity, reducing power assay. In all the methods, the extract combinations exhibited good scavenging activity. Reaction of combination of standardized extracts with 2,2 Diphenyl -1-picryl hydrazyl (DPPH) radicals showed better scavenging activity compared to individual extracts. The IC50 values for TCE+TAE, TCE+OSE, TAE+OSE were found to be 74.12 µg /ml, 78.62 µg /ml and 71.70 µg /ml respectively. The IC50 values for hydroxyl radical scavenging activity of different herb combinations viz., TCE+TAE, TCE+OSE, TAE+OSE were found to be 320.28 µg/ml, 340 µg/ml and 261 µg/ml respectively. Further, the standardized herb combinations showed more effective reductive ability when compared to that of individual standardized herbal extract. Hence, it may be concluded that standardized extracts containing potent antioxidant constituents like phenolics, flavonoids, triterpenoids, alkaloids, steroids, tannins showed synergistic antioxidant activity.
Keywords
Downloads
-
1FullTextPDF
Article
Introduction
Free radicals or reactive oxygen species (ROS) are synthesized in our body due to biological oxidation and participate in different cellular functions including gene expression and apoptosis.1 The excess production of free radicals such as super oxide anion radical, hydroxyl radical, hydrogen peroxide causes damage to different organs and contributes to oxidative stress. Oxidation of lipid, DNA and proteins causes chronic degenerative diseases including hypertension, cancer, diabetes, coronary artery disorders etc. 2,3
Endogenous mechanism protects the organisms from free radicals along with the dietary antioxidants.4 However, synthetic antioxidants like butylated hydroxyanisole and butylated hydroxytoluene have restricted use in foods due to their specific limitations. Hence, the search for safe, natural antioxidants has greatly increased in the recent years. The researchers have now focused on natural antioxidants such as standardized plant extracts and their compounds. These have been recognized to have beneficial effects against ROS in biological systems as antioxidants.5,6
Medicinal plants containing antioxidant constituents such as ascorbic acid, polyphenols, carotenoids, vitamins, and hydrolysable tannins play a vital role in alleviating human diseases.7 Antioxidants are the chemicals which destroy the free radicals. Major plant antioxidants like flavonoid, tannins, phenolics, coumarins, chalcone are mainly biosynthesized via shikimic acid pathway and phenyl-propanoid metabolism8-11.
Tinospora cordifolia (T. cordifolia) (menispermaceae) is a shrub which is extensively used in ayurvedic system and folk medicine as tonic, vitalizer. It is reported to have anti-diabetic, anti-inflammatory, antiallergic, spasmodic, neuroprotective and antioxidant properties.12 Tinosporone, tinosporaside, tinosporic acid, cordifolisides A to E, syringen, giloin, giloinin and, picrotene, bergenin, and arabinogalactan are the major constituents of this plant. Recent studies on standardized extract of T. cordifolia revealed metabolism mediated interaction potential through rat and human liver microsomes, modulation alcohol induced steroid synthesis.13,14
Terminalia arjuna is also known as ‘Arjuna’ and different parts of this plant such as leaves, fruits and bark are extensively used in traditional system of medicine. The stem bark possesses laxative antidysentric, and purgative properties. It is prescribed in the treatment of ulcers, inflammation, tumors, and diarrhea associated with blood as well as documented to have anti-nociceptive and immunomodulatory activities. Moreover, antioxidant activity and cytotoxic in hepatocellular carcinoma cells has been reported with bark extract. The stem bark possesses a substantial amount of secondary metabolites, which may act as resource of pharmacologically active agents and natural antioxidants. Phytochemicals such as gallic acid, ellagic acid, quercetin, which have been reported to be present in T. arjuna bark hold varying degree of antioxidant, protective and intestinal anti-inflammatory effects in rat models of colitis.15,16
Ocimum sanctum (O. sanctum) (the holy basil) belongs to the family Lamiaceae and is reported to possess antioxidant properties. The plant branches out from its base, with angle stems and open foliage. The plant shows aromatic flavor, is pungent and is commonly used for culinary purposes. Within Ayurveda, Ocimum sanctum is said to prevent diseases, promote general health and wellbeing. Previous studies have shown the multitude potential of tulsi including anti-stress, anticonvulsant, anxiolytic and antioxidant activities. Majority of constituents of hydroalcoholic extract of O. sanctum leaves include flavonoids (oleanolic acid, ursolic acid, orientin, and vicenin).17
The anticonvulsant potential of tulsi extracts has been delineated by few previous in vivo studies, conducted in acute seizure models such as maximal electroshock seizure (MES) and pentylenetetrazole (PTZ) model. In one of previous studies, O. sanctum extract has shown 50% protection from MES and PTZ induced seizures.18
Since antioxidant mechanism plays vital role in the treatment of different disorders, the present investigation aims to check the synergistic/antagonistic antioxidant effects of standardised extracts of Tinospora cordifolia (Willd) (TCE), Terminalia arjuna (Roxb) (TAE), Ocimum sanctum (Linn) (OSE) in combinations.
Materials and methods
Chemicals
Standardized extracts of TCS, TAE and OCE were obtained from Sami Labs Limited (19/1, 19/2, I Main, II phase, Peenya Industrial Area, Bangalore, Karnataka-560058) as gift samples. Other chemicals are purchased from SD Fine Chemicals Ltd. (Mumbai, India). All chemicals used in the experiment were of analytical grade.
In vitro free radical scavenging activity
DPPH Scavenging Activity19
The DPPH free radical scavenging activity of diverse combinations of any two standardized extracts (1:1) at different concentrations were measured from bleaching of the purple colour of 2,2 Diphenyl -1-picryl hydrazyl (DPPH). A 3 ml solution of different concentrations of extract was added to 1 ml of DPPH and kept in dark for 30 min. DPPH assay was performed in triplicates. The absorbance of the colour was measured at 517 nm and the percentage of scavenging activity was calculated by using the following equation.
Percentage inhibition (%) = (A0-A1/A0)×100
where:
A0 is the Absorbance of control
A1 Absorbance of test
The results are expressed in terms of IC50 value.
Hydroxyl radical scavenging activity20
Hydroxyl radicals were generated by Fenton reaction (Fe3+-ascorbate-EDTA-H2 O2 system), and the scavenging capacity towards the hydroxyl radicals was measured by using deoxyribose method. The reaction mixture contained 2-deoxy-2-ribose (2.8 mM), phosphate buffer (0.1 mM, pH 7.4), ferric chloride (20 μM), EDTA (100 μM), hydrogen peroxide (500 μM), ascorbic acid (100 μM) and different concentrations (100-600 μg/ml) of the different herb-herb combinations (1:1) in a final volume of 1 ml. The mixture was incubated for 1 h at 37°C. After the incubation, an aliquot of the reaction mixture (0.8 ml) was added to 2.8% TCA solution (1.5 ml), followed by TBA solution (1% in 50 mM sodium hydroxide, 1 ml) and sodium dodecyl sulphate (0.2 ml). The mixture was then heated (20 min at 90°C) to develop the colour. After cooling, the absorbance was measured at 532 nm against an appropriate blank solution. This scavenging activity was performed in triplicates. The percentage of inhibition was expressed according to the following equation:
Percentage inhibition (%) = (A0-A1/A0)×100
where:
A0 is the Absorbance of control
A1 is the Absorbance of test.
The results are expressed in terms of IC50 value.
Reducing Power Assay21
The reducing power of the diverse combinations of any two standardized extracts (1:1) was measured by the method described by Oyaizu.21 An aliquot of extracts (1.0 ml) at different concentrations from 50-450 μg/ml was mixed with phosphate buffer (0.2 M, pH 6.6, 2.5ml) and 1% potassium ferricyanide (2.5 ml, 1%). The mixture was incubated at 50°C for 20 min. After adding 10% trichloroacetic acid (2.5 ml, 10%), the mixture was centrifuged at 6500 rpm for 10 min. The supernatant (2.5 ml) was mixed with distilled water (2.5 ml) and 0.1% iron (III) chloride (0.5 ml) and the absorbance was measured at 700 nm using phosphate buffer as blank. These were done in triplicate and the mean values were given in the results.
Statistical analysis
All the experimental results were expressed in terms of IC50 value, which is the effective concentration at which the antioxidant activity is 50%.
Results
DPPH Scavenging Activity
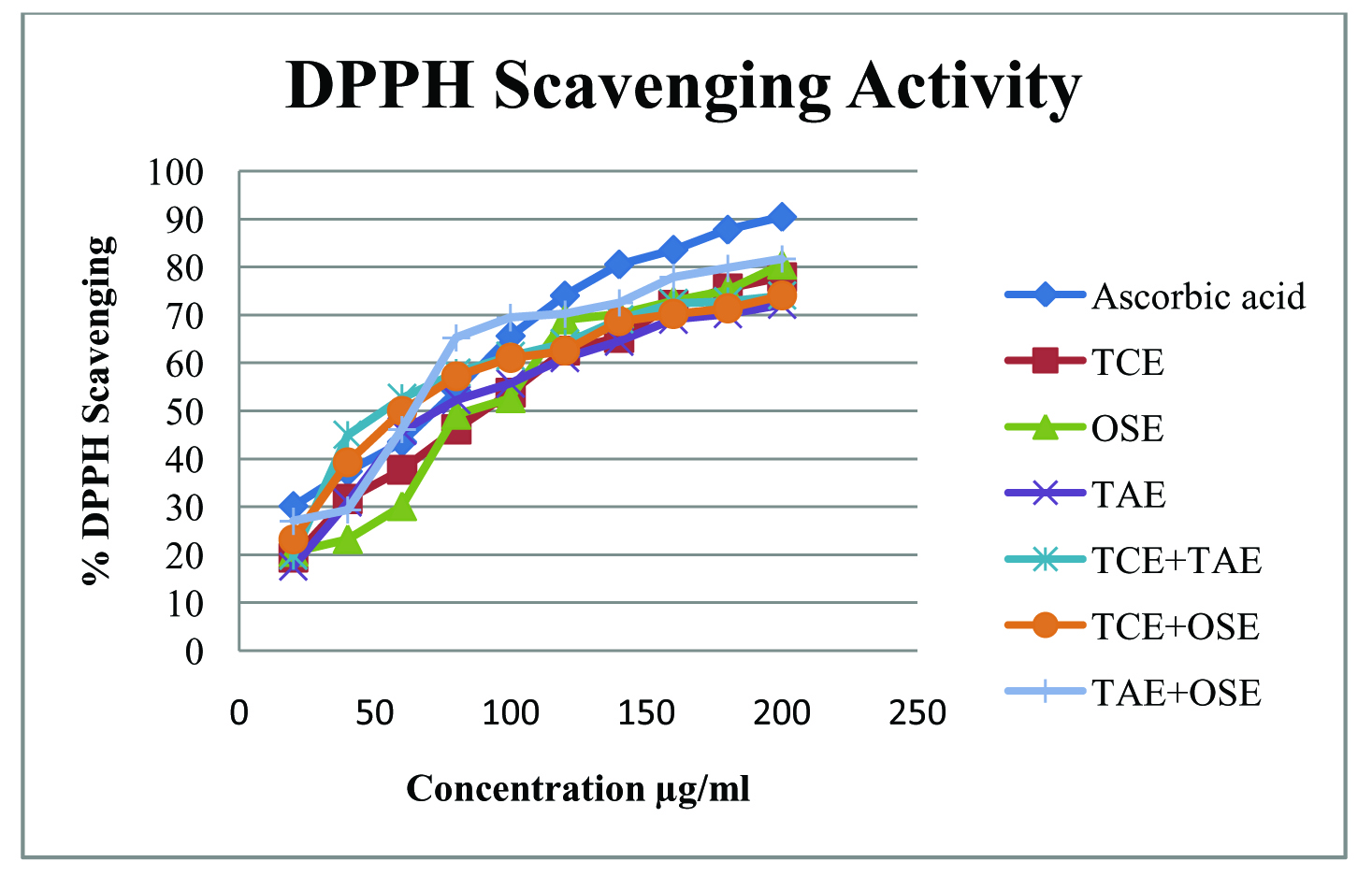
DPPH assay method is mainly based on the reduction of methanolic solution of blue to dark purple coloured free radical DPPH by free radical scavenger. Basically, a higher DPPH radical-scavenging activity is associated with a lower IC50 value. Reaction with DPPH radicals of combination of standardized extracts showed better scavenging activity compared to individual extracts. The IC50 values for TCE+TAE, TCE+OSE, TAE+OSE were found to be 74.12 µg/ml, 78.62 µg/ml and 71.70 µg/ml respectively whereas IC50 value for ascorbic acid was found to be 69.05 μg/ml. A linear correlation coefficient (r2 =0.809, 0.863, 0.853) was obtained (Fig.1). Whereas TCE, OSE, TAE showed IC50 values of 97.09, 98.01, 96.07 respectively with linear correlation coefficient r2 = 0.970, 0.931, 0.889.
Hydroxyl radical scavenging activity
The assay showed the capacity of the extract and standard mannitol to decrease hydroxyl radical-mediated deoxyribose degradation in an Fe3+-EDTA-Mannitol and H2 O2 reaction mixture. The IC50 values for different herb combinations viz., TCE+TAE, TCE+OSE, TAE+OSE were found to be 320.28 µg/ml, 340 µg/ml and 261 µg/ml respectively. A linear correlation coefficient (r2 =0.986, 0.992, 0.936) was obtained (Table 1). The IC50 value for standard mannitol showed 181.65µg/ml with a linear correlation coefficient (r2 =0.927) respectively. Whereas TCE, OSE, TAE showed IC50 values of 399.11, 343.37, 372.78 respectively with linear correlation coefficient r 2 =0.958, 0.997, 0.992.
Reducing Power Assay
Reducing power of individual standardized herbal extract and in combination increased with increase in concentration. The standardized herb combinations viz., TCE+TAE, TCE+OSE, TAE+OSE showed more effective reductive ability when compared to that of individual standardized herbal extract (Fig. 2). Here, Fe (III) reduction is frequently used as a notable indicator of electron donating activity which is a prime mechanism of phenolic antioxidant action by breaking the free radical chain by donating a hydrogen atom.
Discussion
Effect of plant extract combinations or phytochemicals leading stronger effect as compared to individual extracts/compounds at the same concentrations is termed as synergistic effect. Synergistic antioxidant effect was observed with compounds such as vitamins E and C22, vitamin E and β-carotene23, catechin and malvidin-3- glucoside24, tea polyphenols and vitamin E25 and with varieties of fruits.26 In this study, for the first time, we employed to assess synergistic antioxidant effects raised with the combination of standardized extracts of T. cordifolia, T. arjuna and O. sanctum.
Natural polyphenols and carotenoids are the most potent antioxidants in human food, and their free radical scavenging properties are related to substitution of hydroxyl groups in the aromatic rings of phenolics.27 The plant type, geographic location, growing time, and storage condition can all affect the concentrations of polyphenols in food. Dietary polyphenols could be classified into five classes: flavonoids, coumarins, stilbenes, tannins and phenolic acids. Flavonoids are grouped as flavones, flavanols, flavanones, anthocyanidins, and isoflavonoids. Total phenolic content and total antioxidant activity in phytochemical extracts of different herbs may have a direct relationship. When the herbs contain high amount of total phenolic contents, they possess potential antioxidant activity. For example, the scavenging activity of grape seed extract against ABTS radical was strongly linked with the level of phenolic compounds.28
OCE is rich in volatile oil (0.7%), phenolics, flavonoids, neolignans, terpenoids and fatty acid derivatives.29 Caffeic acid, chlorogenic acid, vanillic acid, ocimumnaphthanoic acid and menthylsalicylic glucoside were also isolated from the aerial parts of O. Sanctum.30 Eugenol is one of the most distributed antioxidant phenyl propanoid in the essential oil of O. sanctum leaves. Other phenyl propane derivatives such as ociglycoside or eugenyl-βD-glucoside, citrusin C, ferulaldehyde, bieugenol and dehydrodieugenol were isolated from the leaves of O. Sanctum.31
The major constituents of T. arjuna in stem bark are flavonoids and phenolics like arjunone, luteolin, baicalein, ethyl gallate, gallic acid, kempfero, pelargonidin, quercetin, (+)-catechin, (+)-gallocatechin and (-) -epigallocatechin, gallic acid, ellagic acid and its derivatives which are responsible for its antioxidant activity. T. arjuna also contains triterpenoids; arjunin, arjunic acid, arjungenin, terminic acid, terminoltin, arjunolic acid which further enhances its antioxidant activity.32
T. cordifolia exhibits an antioxidant potential by scavenging the free radicals and ROS. T. cordifolia significantly decreases the regulating lipid peroxidation process and in turn reduces the level of ROS in a diabetic rat model (alloxan induced diabetes). It regulates antioxidant enzymes like glutathione and catalase indicating its antioxidant effects. A clinical research has reported that the extract shows antioxidant effect by raising the level of GSH and reducing the expression of inducible nitric oxide synthase gene. The plant derived polysaccharide compound named as ‘arabinogalactan’ shows protection against free radicals in rat model exhibiting its antioxidant action. In Ayurvedic medicine, Pepticare, a herbomineral formulation which includes T. cordifolia has also been reported to possess potent antioxidant effect in rat model.33
Conclusion
The above discussion revealed that O. sanctum contains phenolics, flavonoids, triterpenoids; whereas, T. arjuna contains triterpenoids, alkaloids, steroids and phenolics, flavonoids, triterpenoids and tannins are present in T. Cordifolia. These potent antioxidant phyto constituents are responsible for synergistic antioxidant activity. Hence, we conclude that combinations of TCE+TAE, TCE+OSE, TAE+OSE have shown synergistic antioxidant activity compared to individual extracts.
Conflicts of interest
None
Acknowledgements
The authors are thankful to the President, Soniya Education Trust and Principal, SET’s College of Pharmacy, Dharwad for encouragement, support and for providing the necessary facilities to carry out the research work. We are also thankful to Sami Labs Limited, Bangalore, Karnataka for providing standardised plant extracts as gift samples.
Supporting File
References
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 2014;24(10):R453-62.
- Yang B, Chen Y, Shi J. Reactive oxygen species (ROS)-based nanomedicine. Chem Rev 2019;119(8):4881-985.
- Premanath R, Lakshmidevi N. Studies on anti-oxidant activity of Tinospora cordifolia (Miers.) leaves using in vitro models. J Ame Sci 2010;6(10):736-743.
- Priftis A, Stagos D, Konstantinopoulos K, Tsitsimpikou C, Spandidos DA, Tsatsakis AM, Tzatzarakis MN, Kouretas D. Comparison of antioxidant activity between green and roasted coffee beans using molecular methods. Mol Med Rep 2015;12(5):7293-7302.
- Reena R, Yuan-Tong Lin MS, Shetty K. Phenolics, their antioxidants and antimicrobial activity in dark germinated fenugreek sprouts in response to peptide and phytochemical elicitors. Asia Pac J Clin Nutr 2004;13(3):295-307.
- Hazra B, Biswas S, Mandal N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complementary Altern Med 2008;8:63-75.
- Durgawale PP, Patil MN, Joshi SA, Korabu KS, Datkhile KD. Studies on phyto constituents, in vitro antioxidant, antibacterial, antiparasitic, antimicrobial, and anticancer potential of medicinal plant Lasiosiphon eriocephalus decne (Family: Thymelaeaceae). J Nat Sci Bio Med 2019;10(1):38.
- Prasanth B, Soman A, Jobin J, Narayanan PS, John AP. Plants and phytoconstituents having sunscreen activity. World J Curr Med Pharm Res 2020;27:14- 20.
- Sharma US, Kumar A. In vitro antioxidant activity of Rubus ellipticus fruits. J Adv Pharm Res 2011;2:47- 50.
- Vadalia DN, Pethe AM, Chakraborthy GS. Antioxidant activity of polyherbal formulation. J Pharm Res 2010;3:1756-1758.
- Pal DK, Mitra S. Preliminary study on the in vitro antioxidant activity of the stems of Opuntia vulgaris. J Adv Pharm Tech Res 2010;1:268-272.
- Singh SS, Pandey SC, Srivastava S, Gupta VS, Patro B. Chemistry and medicinal properties of Tinospora cordifolia (Guduchi). Ind J Pharmacol 2003;35:83- 91.
- Bahadur S, Mukherjee PK, Milan Ahmmed SK, Kar A, Harwansh RK, Pandit S. Metabolism-mediated interaction potential of standardized extract of Tinospora cordifolia through rat and human liver microsomes. Indian J Pharmacol 2016;48(5):576– 581.
- Kumari S, Mittal A, Dabur R. Moderate alcohol consumption in chronic form enhances the synthesis of cholesterol and C-21 steroid hormones, while treatment with Tinospora cordifolia modulate these events in men. Steroids 2016;114:68-77.
- . Pandurangan AK, Mohebali N, Esa NM, Looi CY, Ismail S, Saadatdoust Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int Immunopharmacol 2015;28(2):1034-1043.
- Cota D, Mishra S, Shengule S. Terminalia arjuna hydroalcoholic extract ameliorates trinitrobenzenes ulphonic acid induced colitis mediated through inhibition of inflammation, oxidative stress and improvement in structure of gut microbiota. J Ethnopharmacol 2019;230:117-125.
- Sarangi, SC, Joshi D, Kumar R, Kaleekal T, Gupta YK. Pharmacokinetic and pharmacodynamic interaction of hydroalcoholic extract of Ocimum sanctum with valproate. Epilepsy Behav 2017;75:203–209.
- Sarangi SC, Pattnaik SS, Katyal J, Kaleekal T, Dinda AK. An interaction study of Ocimum sanctum L. and levetiracetam in pentylenetetrazole kindling model of epilepsy. J Ethnopharmacol 2020;249:112389.
- Garba AM, Isa HR, Abubakar S, Ja’afar S. Determination of antioxidant activity of leave extracts of Albizia chevalieri using free radical scavenging activity assay. Int Res J Pure and Appl Chem 2019;30:1-6.
- Halliwell B, Gutteridge JMC, Aruoma OI. The deoxyribose method: A simple ‘test tube’ assay for determination of rates constants for reactions of hydroxyl radical. Anal Biochem 1987;165:215-224.
- Oyaizu M. Studies on products of browning reaction - antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr 1986;4:307-315.
- Scarpa M, Rigo A, Maiorino M, Ursini F, Gregolin C. Formation of α-tocopherol radical and recycling of α-tocopherol by ascorbate during peroxidation of phosphatidylcholine liposomes: an electron paramagnetic resonance study. Biochim Biophys Acta (BBA)-General Subjects. 1984;801(2):215-9.
- Palozza P, Krinsky NI. β-Carotene and α-tocopherol are synergistic antioxidants. Arch Biochem Biophys 1992;297(1):184-187.
- Rossetto M, Vanzani P, Mattivi F, Lunelli M, Scarpa M, Rigo A. Synergistic antioxidant effect of catechin and malvidin 3-glucoside on free radicalinitiated peroxidation of linoleic acid in micelles. Arch Biochem Biophys 2002;408(2):239-45.
- Zhou B, Jia ZS, Chen ZH, Yang L, Wu LM, Liu ZL. Synergistic antioxidant effect of green tea polyphenols with α-tocopherol on free radical initiated peroxidation of linoleic acid in micelles. J Chem Soc Perkin Trans 2000;(4):785-791.
- Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr 2004;134(12):3479S-85S.
- Rokayya S, Li CJ, Zhao Y, Li Y, Sun CH. Cabbage (Brassica oleracea L. var. capitata) phytochemicals with antioxidant and anti-inflammatory potential. Asian Pac J Cancer Prev 2013;14:6657– 6662.
- Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, Li HB. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015;20(12):21138-56.
- Naji-Tabasi S, Razavi SM. Functional properties and applications of basil seed gum: an overview. Food Hydrocoll 2017;73:313-325.
- Skaltsa H, Tzakou O, Singh M. Note polyphenols of Ocimum sanctum from suriname. Pharm Biol 1999;37(1):92-94.
- Kelm MA, Nair MG, Strasburg GM, DeWitt DL. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine 2000;7(1):7-13.
- Amalraj A, Gopi S. Medicinal properties of Terminalia arjuna (Roxb.) Wight & Arn.: a review. J Tradit Complement Med 2017;7(1):65-78.
- Tiwari P, Nayak P, Prusty SK, Sahu PK. Phytochemistry and pharmacology of Tinospora cordifolia: A review. Sys Rev Pharm 2018;9(1):70- 78.