RJPS Vol No: 15 Issue No: 3 eISSN: pISSN:2249-2208
Dear Authors,
We invite you to watch this comprehensive video guide on the process of submitting your article online. This video will provide you with step-by-step instructions to ensure a smooth and successful submission.
Thank you for your attention and cooperation.
1Department of Pharmacology, TMAE Society’s MMJG College of Pharmacy, Haveri, Karnataka, India
2Department of Pharmacology, TMAE Society’s MMJG College of Pharmacy, Haveri, Karnataka, India
3Research Department, TMAE Society’s MMJG College of Pharmacy, Haveri, Karnataka, India
4Narayan R Miskin, Assistant Professor, Department of Pharmacognosy, TMAE Society’s MMJG College of Pharmacy, Haveri, Karnataka, India.
*Corresponding Author:
Narayan R Miskin, Assistant Professor, Department of Pharmacognosy, TMAE Society’s MMJG College of Pharmacy, Haveri, Karnataka, India., Email: nrm_rhm@rediffmail.com1
Abstract
Objective: Bridelia retusa (L.) Spreng., a medicinal plant belonging to the Euphorbiaceae family, is commonly used in India for treating various diseases. The present study investigated the antioxidant potential of different extracts of Bridelia retusa (L.) Spreng. leaves in cell free systems (in vitro).
Method: Dried, coarsely powdered leaves of Bridelia retusa (L.) Spreng. were successively extracted with polarity graded solvents such as petroleum ether, chloroform, ethyl acetate in a Soxhlet apparatus, followed by maceration with water. Antioxidant activity was evaluated using the DPPH (2, 2 diphenyl-1-picrylhydrazyl) and ferric reducing power assays, with ascorbic acid as the standard.
Results: A progressive increase in radical inhibition activity was observed with increasing concentrations of chloroform extract of Bridelia retusa (L.) Spreng., demonstrating effects similar to the standard antioxidant ascorbic acid.
Conclusion: The chloroform extract of Bridelia retusa (L.) Spreng. leaves exhibited significant antioxidant activity comparable to the standard antioxidant.
Keywords
Downloads
-
1FullTextPDF
Article
Introduction
Plants are potent sources of bioactive compounds with notable antioxidant properties. Their secondary metabolites promote health by neutralizing free oxygen radicals and strengthening the body's antioxidant defenses.1 Reactive oxygen and nitrogen species (ROS and RNS) are naturally generated as byproducts of metabolic activities, particularly during the electron transport chain reactions. These highly reactive molecules are produced as part of normal cellular processes, but can accumulate and cause damage if their levels become excessive.2 At low concentrations, reactive oxygen and nitrogen species (ROS and RNS) are vital for various biochemical functions within the body.3
Bridelia retusa (L.) Spreng., commonly referred to as Kasai in India, belongs to Euphorbiaceae family. This plant typically grows as a shrub or small tree, and is characterized by sharp, conical spines that may reach up to 7 cm in length.4 It is also known for the clearance of urinary stones. Both the root and bark serve as effective astringents, and the bark, when combined with sesame oil, is used as a liniment to treat rheumatism.5 Tribal communities use the bark extract of Bridelia retusa as a remedy to induce sterility, utilizing its contraceptive properties.6 In traditional medicine, Bridelia retusa has been utilized for treating conditions such as dysentery, diarrhoea, diabetes, in managing gastrointestinal and metabolic disorders.7 The stem bark extract of Bridelia retusa is known for its antiviral, anticancer, and hypo-tensive properties. A paste made from the bark is applied to wounds, while the juice is administered orally for snake-bite management.8
Free radicals, due to their high reactivity, are notorious for causing oxidative stress in the body. This leads to cellular damage and contributes to aging, inflammation, and numerous chronic diseases. One effective approach to mitigating these harmful effects is by introducing antioxidants, which can neutralize free radicals. While synthetic antioxidants such as Butylated Hydroxy Anisole (BHA), Butylated Hydroxy Toluene (BHT) and TertButylhydroquinone (TBHQ) have been utilized for this purpose, their potential health risks have raised concerns and prompted regulatory restrictions. As a result, there has been a notable shift in scientific research towards exploring natural sources of antioxidants.9 Therefore, the present investigation was essential to explore the phytoconstituents present in the leaves of Bridelia retusa Spreng., and to evaluate their antioxidant effectiveness under in vitro conditions using the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing antioxidant power assays.
Materials and Methods
Drugs and Chemicals
The fresh leaves of Bridelia retusa (L.) Spreng. were carefully identified and collected from Davangere, Karnataka. The identity was verified and authenticated by a qualified taxonomist. The specimens were then air-dried in shaded conditions to preserve the integrity for subsequent analysis.
All chemicals and reagents used in the study were of analytical grade and were procured from Vikas Scientific Supplies, Davangere, and SDFCL, Mumbai.
Extraction
One hundred sixty grams of coarsely powdered, dried leaves of plant Bridelia retusa (L.) Spreng., were subjected to successive extractions using a range of solvents, progressing from non-polar to polar. The extraction process was carried out using petroleum ether, chloroform, and ethyl acetate in a Soxhlet apparatus, followed by maceration with water. The percentage yield of each extract derived from the leaves of the plant was determined.
Qualitative Chemical Analysis
Preliminary phytochemical screening was carried out to assess the presence of various bioactive phytoconstituents in the leaf extracts of the plant. The analysis followed established standard protocols to ensure accurate identification of the compounds.10,11
Alkaloid detection
Extracts were individually dissolved in dilute hydrochloric acid, followed by filtration.
- Mayer’s Test: Reagent which contains potassium mercuric iodide, reacts with alkaloids to form cream or pale-yellow precipitate indicating their presence.
- Wagner’s Test: The reagent, consisting of iodine in potassium iodide, produces a brown or reddish precipitate when alkaloids are present.
- Dragendroff’s Test: The filtrate is mixed with Dragendroff’s reagent (potassium bismuth iodide solution), and the formation of a red precipitate indicates the presence of alkaloids.
- Hager’s Test: The filtrate is mixed with Hager’s reagent (saturated picric acid solution). Formation of a yellow precipitate confirms the presence of alkaloids.
Carbohydrate detection
Five milliliters of distilled water was added to the extracts, which were then filtered, and the resulting filtrates were used for -
- Molisch’s Test: A few drops of Molisch’s reagent (α-naphthol in ethanol) are added to the sample, followed by concentrated sulphuric acid. The appearance of a purple or violet ring at the interface of the two liquids indicates the presence of carbohydrates.
- Benedict’s Test: The filtrate is treated with Benedict’s reagent and gently heated. Formation of an orange-red precipitate indicates the presence of reducing sugars.
- Fehling’s Test: The filtrate is hydrolyzed with dilute hydrochloric acid, neutralized, and then heated with Fehling’s solutions A and B. Formation of a brick-red precipitate indicates the presence of reducing sugars.
Glycoside detection
Extracts were hydrolyzed with dilute HCl and tested.
- Modified Borntrager’s Test: Ferric chloride solution is added to the extract and boiled for five minutes. After cooling, benzene is added and the mixture extracted, followed by treatment with ammonia solution. A rose-pink color in the ammoniacal layer indicates the presence of anthranol glycosides.
- Legal’s Test: The extract is treated with sodium nitroprusside in pyridine and sodium hydroxide. Development of a pink to blood-red color indicates the presence of cardiac glycosides.
Saponin detection
- Froth Test: 20 mL of distilled water is added to the extracts and shaken for 15 minutes. The formation of stable 1 cm foam layer indicates the presence of saponins.
Phytosterol detection
- Salkowski’s Test: The extract is treated with chloroform, followed by concentrated sulphuric acid. Formation of a golden yellow color signifies triterpenes.
- Liebermann-Burchard Test: To a small amount of the extract, a few drops of acetic anhydride is added, followed by the slow addition of concentrated sulphuric acid along the sides of the test tube. The appearance of a green or bluish-green color confirms the presence of steroids, while a reddish to pink color suggests triterpenoids.
Phenol detection
- Ferric Chloride Test: The extract is treated with 3-4 drops of ferric chloride solution. The development of bluish-black color indicates phenols.
Tannin detection
- Gelatin Test: To the extract, 1% gelatin solution containing sodium chloride is added. Development of a white precipitate indicates tannins.
Flavonoid detection
- Alkaline Reagent Test: A few drops of sodium hydroxide solution are added to the plant extract, producing a yellow colour that becomes colorless upon the addition of dilute acid. This reaction indicates the presence of flavonoids.
- Lead Acetate Test: Addition of a few drops of lead acetate solution to the extract produces a yellow precipitate, confirming the presence of flavonoids.
- Shinoda Test: A small amount of the plant extract is dissolved in ethanol and a few fragments of magnesium turnings are added, followed by the dropwise addition of concentrated hydrochloric acid. The appearance of a pink, red, or orange color signifies the presence of flavonoids.
Protein and amino acid detection
- Xanthoproteic Test: The addition of a few drops of concentrated nitric acid to the extracts results in the appearance of a yellow color, indicating the presence of proteins.
- Ninhydrin Test: 0.25% w/v Ninhydrin reagent is added to the extract and boiled for a few minutes. The presence of amino acids shows appearance of blue color.
In Vitro Antioxidant Activity
DPPH assay12 13
Free radical scavenging activity of ethanol-soluble plant extracts was evaluated using 0.1 mM DPPH (2, 2 diphenyl-1-picrylhydrazyl) in ethanol. Extracts at concentrations of 5–30 μg/mL were prepared by serial dilution and mixed with DPPH solution (1 mL DPPH + 3 mL extract). After shaking and standing for 30 minutes at room temperature, absorbance was measured at 517 nm using a UV-VIS spectrophotometer (Shimadzu). Ascorbic acid served as the reference standard and the experiment was conducted in triplicate. Lower absorbance indicated higher scavenging activity. The percent DPPH scavenging effect was calculated using the following formula:
Scavenging Effect (%) = [(A0 - A1) / A0] × 100
Where: A0 is the control absorbance (without sample), A1 is the sample absorbance.
Ferric Reducing Antioxidant Power (FRAP)Assay
The FRAP method of various plant extracts was evaluated as per Yildirim et al. and Lu Foo.14,15 Plant extracts (ranging from 100-200 mg/L) were mixed with phosphate buffer (0.2M, pH 6.6) and potassium ferricyanide (10 g/L), then incubated at 50°C for 20 min. After adding trichloroacetic acid (100 g/L) and centrifuging at 3000 rpm for 10 min, the supernatant was mixed with distilled water and FeCl₃ (1 g/L). Absorbance was measured at 700 nm. Ascorbic acid (5-10 mg/mL) was the standard; phosphate buffer served as the control. A higher absorbance indicates a stronger reducing power, signifying the extract’s ability to donate electrons and reduce ferric ions, similar to the action of ascorbic acid.16
Results
The leaves are simple and elliptic-oblong in shape, with a brown pubescent texture on the underside. They possess 18-25 pairs of lateral veins, typically forked near the edges. The upper surface is green, having a distinct bitter taste (Figure1).
The results of the successive extraction process revealed the following dry weights for each solvent extract. The petroleum ether (40°-60°C) extract (PEBR) yielded 3.4 g, the chloroform extract (CHBR) 5.4 g, the ethyl acetate extract (EABR) 7.2 g, and the aqueous extract (AQBR) 12.4 grams.
Phytochemical screening revealed that carbohydrates, proteins, tannins, and saponin glycosides were present only in the aqueous extract. Alkaloids were detected exclusively in the chloroform extract. Steroids were found in petroleum ether and chloroform extracts, while flavonoids and tannins were identified in the ethyl acetate extract (Table.1)
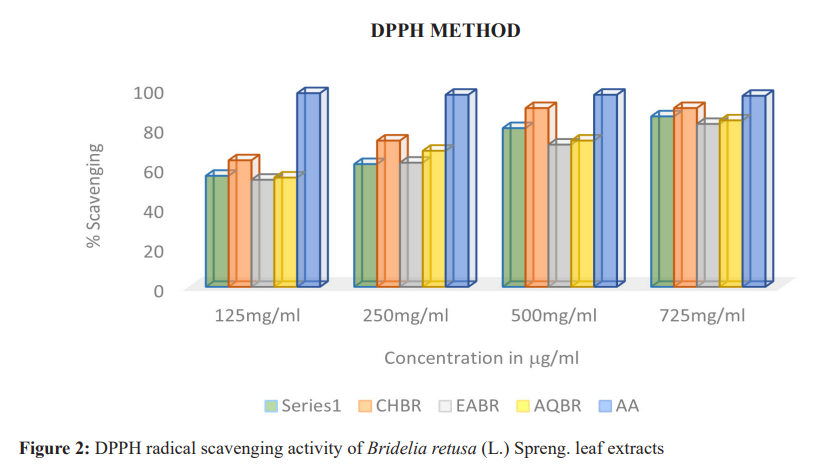
The antioxidant activity of the extracts was evaluated using the DPPH radical scavenging assay. The chloroform extract (CHBR) showed the highest inhibition, reaching 90% at both 500 µg/mL and 750 µg/mL concentrations. Aqueous and ethyl acetate extracts also showed notable activity, though lower than the standard ascorbic acid (Table 2, Figure 2).
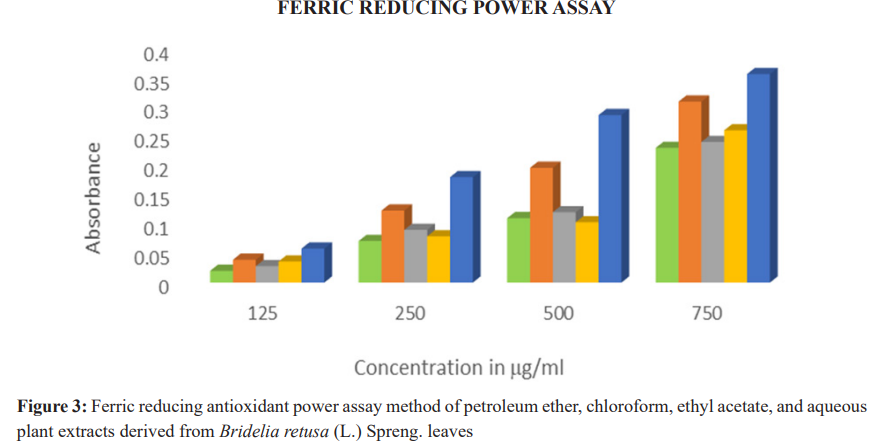
Ferric Reducing Antioxidant Power (FRAP) assay results showed that the chloroform extract had the highest absorbance values across all concentrations, indicating strong reducing power. Aqueous and ethyl acetate extracts followed, while petroleum ether extract showed the least activity (Table 3, Figure 3).
Discussion
The DPPH assay assesses antioxidant activity by measuring the capacity of compounds to scavenge DPPH radicals, indicating their effectiveness in neutralizing free radicals and preventing lipid oxidation. The Ferric Reducing Antioxidant Power (FRAP) assay evaluates the antioxidant potential of a substance based on its ability to convert ferric ions (Fe³⁺) into ferrous ions (Fe²⁺).17 The dried leaves of the plant were successively extracted using the range of solvents like petroleum ether, chloroform, ethyl acetate, and macerated with water. Preliminary phytochemical investigations were determined for all the plant extracts. The petroleum ether extract tested positive for steroids; the chloroform extract contained alkaloids and steroids; the ethyl acetate extract revealed flavonoids and tannins; and the aqueous extract confirmed the presence of carbohydrates, proteins, tannins, and saponin glycosides, respectively.
Antioxidant activity of all extracts was assessed in vitro using the DPPH and ferric reducing power assays. Bridelia retusa (L.) Spreng. leaf chloroform extract demonstrated strongest antioxidant potential in both DPPH radical scavenging and FRAP methods, surpassing the petroleum, ethyl acetate and aqueous extracts. Ascorbic acid served as the reference standard. The observed antioxidant effect of the chloroform extract may be attributed to its phytochemical constituents, including alkaloids and steroids, which are known to exhibit antioxidant effects.
Conclusion
In the current study, Bridelia retusa (L.) Spreng. leaf chloroform extract, rich in phytoconstituents such as alkaloids and steroids, exhibited superior in vitro antioxidant activity compared to petroleum ether, ethyl acetate, and aqueous extracts. These findings suggest its potential in combating oxidative stress, though further studies are needed to isolate and identify the antioxidant compounds within the extract to better understand its mechanisms and potential therapeutic applications.
Conflict of Interest
Nil
Supporting File
References
- Patel RM, Patel NJ. In vitro antioxidant activity of coumarin compounds by DPPH, super oxide and nitric oxide free radical scavenging methods. J Adv Pharm Educ Res 2011;1:52-68.
- Scalbert A, Manach C, Morand C, et al. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci 2005;45:287-306.
- Sakagami H, Satoh K, Hatano T, et al. Possible role of radical intensity and oxidation potential for gallic acid-induced .apoptosis. Anticancer Res 1997;17:377-80.
- Anonymous. The wealth of India. Vol. 2, B. New Delhi: Publications and Information Directorate, CSIR; 1996.
- Kirtikar KR, Basu BD. Indian medicinal plants. 2nd ed. Vol 3. Dehradun, India: International Book Distributors; 1996.
- Jain A, Katewa SS, Gala PK, et al. Medicinal plant diversity of Sitamata wildlife sanctuary, Rajasthan. India. J Ethnopharmacol 2005;102:143-57.
- Raju SV, Reddy KN. Ethnomedicine for dysentery and diarrhoea from Khammam district of Andhra Pradesh. Indian Journal of Traditional Knowledge 2005;4(4):443-7.
- Pawar S, Patil DA. Observations on folkloric medicinal plants of Jalgaon district, Maharashtra. Indian Journal of Traditional Knowledge 2004;3(4):437-42.
- Kumar NS, Nusrath A, Ramadas D. In vitro antioxidant activity of crude protein of Coleus aromaticus. RJMS 2020;9(1):22-7.
- Brain KR, Turner TD. The practical evaluation of pharmaceuticals. 2nded.Bristol:Wright Sciencechnia; 1975.
- Khandelwal KR. Practical pharmacognosy. 9th ed. Delhi: Nirali Prakashan; 2002.
- Rashed K, Butnariu M. Antimicrobial and antioxidant activities of Bauhinia racemosa Lam. and chemical content. Iran J Pharm Res 2014;13(3):1073-80.
- Sashidhara KV, Singh SP, Srivastava A, et al. Main extracts and hypolipidemic effects of the Bauhinia racemosa Lam. leaf extract in HFD-fed hamsters. Nat Prod Res 2013;27(12):1127-31.
- Yildirim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem 2001;49:4083-9.
- Lu Y, Foo Y. Antioxidant activities of polyphenols from sage (Salvia officinalis). Food Chem 2000;75: 197-202.
- Siju EN, Rajalakshmi GR, Kavitha VP, et al. In vitro antioxidant activity of Mussaenda Frondos. Int J Pharm Tech Res 2010;2:1236-40.
- Muthoni Guchu B, Machocho AK, Mwihia SK, et al. In Vitro antioxidant activities of methanolic extracts of Caesalpinia volkensii Harms., Vernonia lasiopus O. Hoffm., and Acacia hockii De Wild. Evid Based Complement Alternat Med 2020;2020:3586268.